Science 101 BIOLOGY
Monday, August 24, 2009
Human Anatomy & Physiology of the Respiratory System
We often complete the daily tasks of living without thinking about the respiratory system. We breathe in and out and take for granted one of our most vital organ systems. The respiratory system provides the oxygen necessary to sustain life. It consists of both upper and lower respiratory tracts. It is divided into two functions: conducting and respiration.
Function
1. The function of the respiratory system is to give us a surface area for
exchanging gases between the air and our circulating blood. It moves that
air to and from the surfaces of the lungs while it protects the lungs from
dehydration, temperature changes and unwelcome pathogens. It also plays a
part in making sounds such as talking, singing, other nonverbal sounds and
works with the central nervous system for the ability to smell.
Upper Respiratory Anatomy
2. The upper respiratory system consists of the nostrils (external nares),
nasal cavity, nasal vestibule, nasal septum, both hard and soft palate,
nasopharynx, pharynx, larynx and trachea. Within the nostrils, course hairs
protect us from dust, insects and sand. The hard palate serves to separate
the oral and nasal cavities. There is a protective mucous membrane that lines
the naval cavities and other parts of the respiratory tract. It is secreted
over the exposed surfaces and then the cilia sweeps that mucus and any
microorganisms or debris to the pharynx, so it is swallowed and then
destroyed in stomach acids.
Lower Respiratory Anatomy
3. The trachea branches off into what is known as the bronchi (more commonly
called bronchial tubes). These two main bronchi have branches forming the
bronchial tree. Where it enters the lung, there is then secondary bronchi.
In each lung, the secondary bronchi divides into tertiary bronchi and in turn
these divide repeatedly into smaller bronchioles. The bronchioles control the
ratio of resistance to airflow and distribution of air in our lungs. The
bronchioles open into the alveolar ducts. Alveolar sacs are at the end of the
ducts. These sacs are chambers that are connected to several individual
alveoli, which makes up the exchange surface of the lungs.
The Lungs
4. The human respiratory system has two lungs, which contain lobes separated
by deep fissures. Surprisingly, the right lung has three lobes while the
left one has only two lobes. The lungs are made up of elastic fibers that
gives it the ability to handle large changes in air volume. The pleural cavity
is where the lungs are located. The diaphragm is the muscle that makes up the
floor of the thoracic cavity and plays a major role in the pressure and volume
of air moving in and out of the lungs.
Significance
5. Our lungs filter and deliver oxygen that is necessary for healthy red blood
cells. It is important that we keep the respiratory tract healthy through
proper rest, hydration, diet and exercise.
How to Tell a Monocot From a Dicot
Plants that produce seeds are put into two categories: monocots and dicots. This distinction is more than just a bit of scientific trivia. The difference between monocots and dicots has been exploited by manufacturers of weed killers. Their chemicals are made to target dicots, not monocots. Since grass is a monocot and most weeds are dicots, this works out very well for our lawns. Knowing how to tell a monocot from a dicot will let you predict which plants will be affected by your lawn sprays. Read on to learn how to tell a monocot from a dicot.
Instructions
Step 1
Observe the sprouts and look at the cotyledons. The “cot” in monocot and
dicot is short for “cotyledon.” The cotyledon is the “seed leaf” that the
seed puts out when it sprouts. A monocot has a single seed leaf (“mono” means
“one”) and a dicot has two seed leaves (“di” means “two”). This observation
can only be made when the seed is just sprouting.
Step 2
Observe the roots. Monocots have short fibrous roots that stay close to the
surface. Dicots have a long, central tap root that goes deep into the ground.
Dicots may also have other roots, too, surrounding the tap root.
Step 3
Observe the veins in the leaves. The veins in monocots run parallel to each
other, as seen in grass leaves. Monocot leaves tend to be long and narrow.
The veins in dicot leaves branch out like the veins in your hands.
Step 4
Observe the petals of the flowers. Monocot petals come in multiples of three
such as three, six or nine. Dicots petals and sepals come in multiples of
four or five--four, eight and 12 or five, 10 and 15.
Step 5
Observe a cross section of the stem under a microscope. Monocots have their
vascular bundles scattered randomly throughout. Dicots have their vascular
bundles arranged neatly in a circle, as if they were placed on imaginary
spokes coming out of the center.
Step 6
Monocots mainly belong to the grass family of plants. Examples of monocots
are grass, wheat, oats, barley, corn, rice, bamboo, onion, asparagus, lilies,
bananas and palm trees.
Step 7
Dicots are mainly broad leaf trees, ornamental flowers, and fruits and
vegetables. Examples of dicots include maple and oak trees, fruit trees,
grapes, strawberries, daisies, marigolds, roses and garden vegetables such
as tomatoes, squash, beans, peas and potatoes.
Tuesday, August 18, 2009
The Human Digestive System
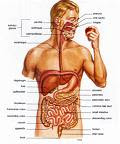
The Human Digestive System
If a human adult’s digestive tract were stretched out, it would be 6 to 9 m (20 to 30 ft) long. In humans, digestion begins in the mouth, where both mechanical and chemical digestion occur. The mouth quickly converts food into a soft, moist mass. The muscular tongue pushes the food against the teeth, which cut, chop, and grind the food. Glands in the cheek linings secrete mucus, which lubricates the food, making it easier to chew and swallow. Three pairs of glands empty saliva into the mouth through ducts to moisten the food. Saliva contains the enzyme ptyalin, which begins to hydrolyze (break down) starch—a carbohydrate manufactured by green plants.
Once food has been reduced to a soft mass, it is ready to be swallowed. The tongue pushes this mass—called a bolus—to the back of the mouth and into the pharynx. This cavity between the mouth and windpipe serves as a passageway both for food on its way down the alimentary canal and for air passing into the windpipe. The epiglottis, a flap of cartilage, covers the trachea (windpipe) when a person swallows. This action of the epiglottis prevents choking by directing food from the windpipe and toward the stomach.
A The Esophagus
The presence of food in the pharynx stimulates swallowing, which squeezes the food into the esophagus. The esophagus, a muscular tube about 25 cm (10 in) long, passes behind the trachea and heart and penetrates the diaphragm (muscular wall between the chest and abdomen) before reaching the stomach. Food advances through the alimentary canal by means of rhythmic muscle contractions (tightenings) known as peristalsis. The process begins when circular muscles in the esophagus wall contract and relax (widen) one after the other, squeezing food downward toward the stomach. Food travels the length of the esophagus in two to three seconds.
A circular muscle called the esophageal sphincter separates the esophagus and the stomach. As food is swallowed, this muscle relaxes, forming an opening through which the food can pass into the stomach. Then the muscle contracts, closing the opening to prevent food from moving back into the esophagus. The esophageal sphincter is the first of several such muscles along the alimentary canal. These muscles act as valves to regulate the passage of food and keep it from moving backward.
Inorganic compound
Traditionally, inorganic compounds are considered to be of a mineral, not biological, origin. Complementarily, most organic compounds are traditionally viewed as being of biological origin. Over the past century, the precise classification of inorganic vs organic compounds has become less important to scientists, primarily because the majority of known compounds are synthetic and not of natural origin. Furthermore, most compounds considered the purview of modern inorganic chemistry contain organic ligands. The fields of organometallic chemistry and bioinorganic chemistry explicitly focus on the areas between the fields of organic, biological, and inorganic chemistry.
Inorganic compounds can be formally defined with reference to what they are not—organic compounds. Organic compounds are those which contain carbon, although some carbon-containing compounds are traditionally considered inorganic. When considering inorganic chemistry and life, it is useful to recall that many species in nature are not compounds per se but are ions. Sodium, chloride, and phosphate ions are essential for life, as are some inorganic molecules such as carbonic acid, nitrogen, carbon dioxide, water and oxygen. Aside from these simple ions and molecules, virtually all species covered by bioinorganic chemistry contain carbon and can be considered organic or organometallic.
Many compounds that contain carbon, are considered inorganic; for example, carbon monoxide, carbon dioxide, carbonates, cyanides, cyanates, carbides, and thyocyanates. In general, however, the workers in these areas are not concerned about strict definitions.
Organic compound
Organic compounds are those that are not composed of carbon, hydrogen and oxygen.
Methane is one of the simplest organic compounds. An organic compound is any member of a large class of chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of compounds such as carbonates, simple oxides of carbon and cyanides, as well as the allotropes of carbon, are considered inorganic. The division between "organic" and "inorganic" carbon compounds while "useful in organizing the vast subject of chemistry...is somewhat arbitrary".
Organic chemistry is the science concerned with all aspects of organic compounds. Organic synthesis is the methodology of their preparation.
Methane is one of the simplest organic compounds. An organic compound is any member of a large class of chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of compounds such as carbonates, simple oxides of carbon and cyanides, as well as the allotropes of carbon, are considered inorganic. The division between "organic" and "inorganic" carbon compounds while "useful in organizing the vast subject of chemistry...is somewhat arbitrary".
Organic chemistry is the science concerned with all aspects of organic compounds. Organic synthesis is the methodology of their preparation.
Sunday, August 16, 2009
Amino acid
The general structure of an α-amino acid, with the amino group on the left and the carboxyl group on the rightIn chemistry, an amino acid is a molecule containing both amine and carboxyl functional groups. These molecules are particularly important in biochemistry, where this term refers to alpha-amino acids with the general formula H2NCHRCOOH, where R is an organic substituent. In the alpha amino acids, the amino and carboxylate groups are attached to the same carbon atom, which is called the α–carbon. The various alpha amino acids differ in which side chain (R group) is attached to their alpha carbon. They can vary in size from just a hydrogen atom in glycine through a methyl group in alanine to a large heterocyclic group in tryptophan.
Amino acids are critical to life, and have a variety of roles in metabolism. One particularly important function is as the building blocks of proteins, which are linear chains of amino acids. Amino acids are also important in many other biological molecules, such as forming parts of coenzymes, as in S-adenosylmethionine, or as precursors for the biosynthesis of molecules such as heme. Due to this central role in biochemistry, amino acids are very important in nutrition.
Amino acids are commonly used in food technology and industry. For example, monosodium glutamate is a common flavor enhancer that gives foods the taste called umami. Beyond the amino acids that are found in all forms of life, amino acids are also used in industry. Applications include the production of biodegradable plastics, drugs and chiral catalysts.
Essential Nonessential
Isoleucine Alanine
Leucine Asparagine
Lysine Aspartate
Methionine Cysteine*
Phenylalanine Glutamate
Threonine Glutamine*
Tryptophan Glycine*
Valine Proline*
Serine*
Tyrosine*
Arginine*
Histidine*
Amino acids are critical to life, and have a variety of roles in metabolism. One particularly important function is as the building blocks of proteins, which are linear chains of amino acids. Amino acids are also important in many other biological molecules, such as forming parts of coenzymes, as in S-adenosylmethionine, or as precursors for the biosynthesis of molecules such as heme. Due to this central role in biochemistry, amino acids are very important in nutrition.
Amino acids are commonly used in food technology and industry. For example, monosodium glutamate is a common flavor enhancer that gives foods the taste called umami. Beyond the amino acids that are found in all forms of life, amino acids are also used in industry. Applications include the production of biodegradable plastics, drugs and chiral catalysts.
Essential Nonessential
Isoleucine Alanine
Leucine Asparagine
Lysine Aspartate
Methionine Cysteine*
Phenylalanine Glutamate
Threonine Glutamine*
Tryptophan Glycine*
Valine Proline*
Serine*
Tyrosine*
Arginine*
Histidine*
Lipids
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The main biological functions of lipids include energy storage, as structural components of cell membranes, and as important signaling molecules.
Lipids may be broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids and polyketides (derived from condensation of ketoacyl subunits); and sterol lipids and prenol lipids (derived from condensation of isoprene subunits).
Although the term lipid is sometimes used as a synonym for fats, fats are a subgroup of lipids called triglycerides. Lipids also encompass molecules such as fatty acids and their derivatives (including tri-, di-, and monoglycerides and phospholipids), as well as other sterol-containing metabolites such as cholesterol. Although humans and other mammals use various biosynthetic pathways to both break down and synthesize lipids, some essential lipids cannot be made this way and must be obtained from the diet.
Fatty acyls
Fatty acyls, a generic term for describing fatty acids, their conjugates and derivatives, are a diverse group of molecules synthesized by chain-elongation of an acetyl-CoA primer with malonyl-CoA or methylmalonyl-CoA groups in a process called fatty acid synthesis. They are made of a hydrocarbon chain that terminates with a carboxylic acid group; this arrangement confers the molecule with a polar, hydrophilic end, and a nonpolar, hydrophobic end that is insoluble in water. The fatty acid structure is one of the most fundamental categories of biological lipids, and is commonly used as a building block of more structurally complex lipids. The carbon chain, typically between four to 24 carbons long, may be saturated or unsaturated, and may be attached to functional groups containing oxygen, halogens, nitrogen and sulfur. Where a double bond exists, there is the possibility of either a cis or trans geometric isomerism, which significantly affects the molecule's molecular configuration. Cis-double bounds cause the fatty acid chain to bend, an effect that is more pronounced the more double bonds there are in a chain. This in turn plays an important role in the structure and function of cell membranes. Most naturally occurring fatty acids are of the cis configuration, although the trans form does exist in some natural and partially hydrogenated fats and oils.
Examples of biologically important fatty acids are the eicosanoids, derived primarily from arachidonic acid and eicosapentaenoic acid, which include prostaglandins, leukotrienes, and thromboxanes. Other major lipid classes in the fatty acid category are the fatty esters and fatty amides. Fatty esters include important biochemical intermediates such as wax esters, fatty acid thioester coenzyme A derivatives, fatty acid thioester ACP derivatives and fatty acid carnitines. The fatty amides include N-acyl ethanolamines, such as the cannabinoid neurotransmitter anandamide.
Glycerolipids
Glycerolipids are composed mainly of mono-, di- and tri-substituted glycerols, the most well-known being the fatty acid esters of glycerol (triacylglycerols), also known as triglycerides. In these compounds, the three hydroxyl groups of glycerol are each esterified, usually by different fatty acids. Because they function as a food store, these lipids comprise the bulk of storage fat in animal tissues. The hydrolysis of the ester bonds of triacylglycerols and the release of glycerol and fatty acids from adipose tissue is called fat mobilization.
Additional subclasses of glycerolipids are represented by glycosylglycerols, which are characterized by the presence of one or more sugar residues attached to glycerol via a glycosidic linkage. Examples of structures in this category are the digalactosyldiacylglycerols found in plant membranes and seminolipid from mammalian sperm cells.
Glycerophospholipids
Glycerophospholipids, also referred to as phospholipids, are ubiquitous in nature and are key components of the lipid bilayer of cells, as well as being involved in metabolism and cell signaling. Neural tissue (including the brain) contains relatively high amounts of glycerophospholipids, and alterations in their composition has been implicated in various neurological disorders. Glycerophospholipids may be subdivided into distinct classes, based on the nature of the polar headgroup at the sn-3 position of the glycerol backbone in eukaryotes and eubacteria, or the sn-1 position in the case of archaebacteria.
Phosphatidylethanolamine (Examples of glycerophospholipids found in biological membranes are phosphatidylcholine (also known as PC, GPCho or lecithin), phosphatidylethanolamine (PE or GPEtn) and phosphatidylserine (PS or GPSer). In addition to serving as a primary component of cellular membranes and binding sites for intra- and intercellular proteins, some glycerophospholipids in eukaryotic cells, such as phosphatidylinositols and phosphatidic acids are either precursors of, or are themselves, membrane-derived second messengers. Typically, one or both of these hydroxyl groups are acylated with long-chain fatty acids, but there are also alkyl-linked and 1Z-alkenyl-linked (plasmalogen) glycerophospholipids, as well as dialkylether variants in archaebacteria.
Sphingolipids
Sphingolipids are a complex family of compounds that share a common structural feature, a sphingoid base backbone that is synthesized de novo from the amino acid serine and a long-chain fatty acyl CoA, then converted into ceramides, phosphosphingolipids, glycosphingolipids and other compounds. The major sphingoid base of mammals is commonly referred to as sphingosine. Ceramides (N-acyl-sphingoid bases) are a major subclass of sphingoid base derivatives with an amide-linked fatty acid. The fatty acids are typically saturated or mono-unsaturated with chain lengths from 16 to 26 carbon atoms.
Sphingomyelin.The major phosphosphingolipids of mammals are sphingomyelins (ceramide phosphocholines), whereas insects contain mainly ceramide phosphoethanolamines and fungi have phytoceramide phosphoinositols and mannose-containing headgroups. The glycosphingolipids are a diverse family of molecules composed of one or more sugar residues linked via a glycosidic bond to the sphingoid base. Examples of these are the simple and complex glycosphingolipids such as cerebrosides and gangliosides.
Sterol lipids
Sterol lipids, such as cholesterol and its derivatives, are an important component of membrane lipids, along with the glycerophospholipids and sphingomyelins. The steroids, all derived from the same fused four-ring core structure, have different biological roles as hormones and signaling molecules. The eighteen-carbon (C18) steroids include the estrogen family whereas the C19 steroids comprise the androgens such as testosterone and androsterone. The C21 subclass includes the progestogens as well as the glucocorticoids and mineralocorticoids. The secosteroids, comprising various forms of vitamin D, are characterized by cleavage of the B ring of the core structure. Other examples of sterols are the bile acids and their conjugates, which in mammals are oxidized derivatives of cholesterol and are synthesized in the liver. The plant equivalents are the phytosterols, such as β-sitosterol, stigmasterol, and brassicasterol; the latter compound is also used as a biomarker for algal growth. The predominant sterol in fungal cell membranes is ergosterol.
Prenol lipids
Prenol lipids are synthesized from the 5-carbon precursors isopentenyl diphosphate and dimethylallyl diphosphate that are produced mainly via the mevalonic acid (MVA) pathway. The simple isoprenoids (linear alcohols, diphosphates, etc.) are formed by the successive addition of C5 units, and are classified according to number of these terpene units. Structures containing greater than 40 carbons are known as polyterpenes. Carotenoids are important simple isoprenoids that function as antioxidants and as precursors of vitamin A. Another biologically important class of molecules is exemplified by the quinones and hydroquinones, which contain an isoprenoid tail attached to a quinonoid core of non-isoprenoid origin.Vitamin E and vitamin K, as well as the ubiquinones, are examples of this class. Prokaryotes synthesize polyprenols (called bactoprenols) in which the terminal isoprenoid unit attached to oxygen remains unsaturated, whereas in animal polyprenols (dolichols) the terminal isoprenoid is reduced.
Saccharolipids
Structure of the saccharolipid Kdo2-Lipid A. Glucosamine residues in blue, Kdo residues in red, acyl chains in black and phosphate groups in green.Saccharolipids describe compounds in which fatty acids are linked directly to a sugar backbone, forming structures that are compatible with membrane bilayers. In the saccharolipids, a monosaccharide substitutes for the glycerol backbone present in glycerolipids and glycerophospholipids. The most familiar saccharolipids are the acylated glucosamine precursors of the Lipid A component of the lipopolysaccharides in Gram-negative bacteria. Typical lipid A molecules are disaccharides of glucosamine, which are derivatized with as many as seven fatty-acyl chains. The minimal lipopolysaccharide required for growth in E. coli is Kdo2-Lipid A, a hexa-acylated disaccharide of glucosamine that is glycosylated with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues.
Polyketides
Polyketides are synthesized by polymerization of acetyl and propionyl subunits by classic enzymes as well as iterative and multimodular enzymes that share mechanistic features with the fatty acid synthases. They comprise a large number of secondary metabolites and natural products from animal, plant, bacterial, fungal and marine sources, and have great structural diversity. Many polyketides are cyclic molecules whose backbones are often further modified by glycosylation, methylation, hydroxylation, oxidation, and/or other processes. Many commonly used anti-microbial, anti-parasitic, and anti-cancer agents are polyketides or polyketide derivatives, such as erythromycins, tetracyclines, avermectins, and antitumor epothilones.
Biological functions
Membranes
Eukaryotic cells are compartmentalized into membrane-bound organelles which carry out different biological functions. The glycerophospholipids are the main structural component of biological membranes, such as the cellular plasma membrane and the intracellular membranes of organelles; in animal cells the plasma membrane physically separates the intracellular components from the extracellular environment. The glycerophospholipids are amphipathic molecules (containing both hydrophobic and hydrophilic regions) that contain a glycerol core linked to two fatty acid-derived "tails" by ester linkages and to one "head" group by a phosphate ester linkage. While glycerophospholipids are the major component of biological membranes, other non-glyceride lipid components such as sphingomyelin and sterols (mainly cholesterol in animal cell membranes) are also found in biological membranes.In plants and algae, the galactosyldiacylglycerols, and sulfoquinovosyldiacylglycerol,which lack a phosphate group, are important components of membranes of chloroplasts and related organelles and are the most abundant lipids in photosynthetic tissues, including those of higher plants, algae and certain bacteria.
Bilayers have been found to exhibit high levels of birefringence which can be used to probe the degree of order (or disruption) within the bilayer using techniques such as dual polarisation interferometry
Self-organization of phospholipids: a spherical liposome, a micelle and a lipid bilayer.A biological membrane is a form of lipid bilayer. The formation of lipid bilayers is an energetically-preferred process when the glycerophospholipids described above are in an aqueous environment. In an aqueous system, the polar heads of lipids align towards the polar, aqueous environment, while the hydrophobic tails minimize their contact with water and tend to cluster together, forming a vesicle; depending on the concentration of the lipid, this biophysical interaction may result in the formation of micelles, liposomes, or lipid bilayers. Other aggregations are also observed and form part of the polymorphism of amphiphile (lipid) behavior. Phase behavior is an area of study within biophysics and is the subject of current academic research. Micelles and bilayers form in the polar medium by a process known as the hydrophobic effect. When dissolving a lipophilic or amphiphilic substance in a polar environment, the polar molecules (i.e., water in an aqueous solution) become more ordered around the dissolved lipophilic substance, since the polar molecules cannot form hydrogen bonds to the lipophilic areas of the amphiphile. So in an aqueous environment, the water molecules form an ordered "clathrate" cage around the dissolved lipophilic molecule.
Energy storage
Triacylglycerols, stored in adipose tissue, are a major form of energy storage in animals. The adipocyte, or fat cell, is designed for continuous synthesis and breakdown of triacylglycerols, with breakdown controlled mainly by the activation of hormone-sensitive enzyme lipase.[46] The complete oxidation of fatty acids provides high caloric content, about 9 kcal/g, compared with 4 kcal/g for the breakdown of carbohydrates and proteins. Migratory birds that must fly long distances without eating use stored energy of triacylglycerols to fuel their flights.[47]
Signaling
In recent years, evidence has emerged showing that lipid signaling is a vital part of the cell signaling. Lipid signaling may occur via activation of G protein-coupled or nuclear receptors, and members of several different lipid categories have been identified as signaling molecules and cellular messengers. These include sphingosine-1-phosphate, a sphingolipid derived from ceramide that is a potent messenger molecule involved in regulating calcium mobilization, cell growth, and apoptosis; diacylglycerol (DAG) and the phosphatidylinositol phosphates (PIPs), involved in calcium-mediated activation of protein kinase C; the prostaglandins, which are one type of fatty-acid derived eicosanoid involved in inflammation and immunity; the steroid hormones such as estrogen, testosterone and cortisol, which modulate a host of functions such as reproduction, metabolism and blood pressure; and the oxysterols such as 25-hydroxy-cholesterol that are liver X receptor agonists.
Other functions
The "fat-soluble" vitamins (A, D, E and K) – which are isoprene-based lipids – are essential nutrients stored in the liver and fatty tissues, with a diverse range of functions. Acyl-carnitines are involved in the transport and metabolism of fatty acids in and out of mitochondria, where they undergo beta oxidation. Polyprenols and their phosphorylated derivatives also play important transport roles, in this case the transport of oligosaccharides across membranes. Polyprenol phosphate sugars and polyprenol diphosphate sugars function in extra-cytoplasmic glycosylation reactions, in extracellular polysaccharide biosynthesis (for instance, peptidoglycan polymerization in bacteria), and in eukaryotic protein N-glycosylation. Cardiolipins are a subclass of glycerophospholipids containing four acyl chains and three glycerol groups that are particularly abundant in the inner mitochondrial membrane.They are believed to activate enzymes involved with oxidative phosphorylation.
Metabolism
The major dietary lipids for humans and other animals are animal and plant triglycerides, sterols, and membrane phospholipids. The process of lipid metabolism synthesizes and degrades the lipid stores and produces the structural and functional lipids characteristic of individual tissues.
Biosynthesis
In animals, when there is an oversupply of dietary carbohydrate, the excess carbohydrate is converted to triacylglycerol. This involves the synthesis of fatty acids from acetyl-CoA and the esterification of fatty acids in the production of triacylglycerol, a process called lipogenesis.Fatty acids are made by fatty acid synthases that polymerize and then reduce acetyl-CoA units. The acyl chains in the fatty acids are extended by a cycle of reactions that add the acetyl group, reduce it to an alcohol, dehydrate it to an alkene group and then reduce it again to an alkane group. The enzymes of fatty acid biosynthesis are divided into two groups, in animals and fungi all these fatty acid synthase reactions are carried out by a single multifunctional protein,while in plant plastids and bacteria separate enzymes perform each step in the pathway. The fatty acids may be subsequently converted to triacylglycerols that are packaged in lipoproteins and secreted from the liver.
The synthesis of unsaturated fatty acids involves a desaturation reaction, whereby a double bond is introduced into the fatty acyl chain. For example, in humans, the desaturation of stearic acid by stearoyl-CoA desaturase-1 produces oleic acid. The doubly-unsaturated fatty acid linoleic acid as well as the triply-unsaturated linolenic acid cannot be synthesized in mammalian tissues, and are therefore essential fatty acids and must be obtained from the diet.
Triacylglycerol synthesis takes place in the endoplasmic reticulum by metabolic pathways in which acyl groups in fatty acyl-CoAs are transferred to the hydroxyl groups of glycerol-3-phosphate and diacylglycerol.
Terpenes and isoprenoids, including the carotenoids, are made by the assembly and modification of isoprene units donated from the reactive precursors isopentenyl pyrophosphate and dimethylallyl pyrophosphate. These precursors can be made in different ways. In animals and archaea, the mevalonate pathway produces these compounds from acetyl-CoA,while in plants and bacteria the non-mevalonate pathway uses pyruvate and glyceraldehyde 3-phosphate as substrates. One important reaction that uses these activated isoprene donors is steroid biosynthesis. Here, the isoprene units are joined together to make squalene and then folded up and formed into a set of rings to make lanosterol. Lanosterol can then be converted into other steroids such as cholesterol and ergosterol.
Degradation
Beta oxidation is the metabolic process by which fatty acids are broken down in the mitochondria and/or in peroxisomes to generate acetyl-CoA. For the most part, fatty acids are oxidized by a mechanism that is similar to, but not identical with, a reversal of the process of fatty acid synthesis. That is, two-carbon fragments are removed sequentially from the carboxyl end of the acid after steps of dehydrogenation, hydration, and oxidation to form a beta-keto acid, which is split by thiolysis. The acetyl-CoA is then ultimately converted into ATP, CO2, and H2O using the citric acid cycle and the electron transport chain. The energy yield of the complete oxidation of the fatty acid palmitate is 106 ATP. Unsaturated and odd-chain fatty acids require additional enzymatic steps for degradation.
Nutrition and health
Most of the lipid found in food is in the form of triacylglycerols, cholesterol and phospholipids. A minimum amount of dietary fat is necessary to facilitate absorption of fat-soluble vitamins (A, D, E and K) and carotenoids.[72] Humans and other mammals have a dietary requirement for certain essential fatty acids, such as linoleic acid (an omega-6 fatty acid) and alpha-linolenic acid (an omega-3 fatty acid) because they cannot be synthesized from simple precursors in the diet.Both of these fatty acids are 18-carbon polyunsaturated fatty acids differing in the number and position of the double bonds. Most vegetable oils are rich in linoleic acid (safflower, sunflower, and corn oils). Alpha-linolenic acid is found in the green leaves of plants, and in selected seeds, nuts and legumes (particularly flax, rapeseed, walnut and soy).[74] Fish oils are particularly rich in the longer-chain omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). A large number of studies have shown positive health benefits associated with consumption of omega-3 fatty acids on infant development, cancer, cardiovascular diseases, and various mental illnesses, such as depression, attention-deficit hyperactivity disorder, and dementia.In contrast, it is now well-established that consumption of trans fats, such as those present in partially hydrogenated vegetable oils, are a risk factor for cardiovascular disease.
A few studies have suggested that total diary fat intake is linked to an increased risk of obesity and diabetes.However, a number of very large studies, including the Women's Health Initiative Dietary Modification Trial, an eight year study of 49,000 women, the Nurses' Health Study and the Health Professionals Follow-up Study, revealed no such links.None of these studies suggested any connection between percentage of calories from fat and risk of cancer, heart disease or weight gain. The Nutrition Source, a website maintained by the Department of Nutrition at the Harvard School of Public Health, summarizes the current evidence on the impact of dietary fat: "Detailed research—much of it done at Harvard—shows that the total amount of fat in the diet isn't really linked with weight or disease."
Lipids may be broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids and polyketides (derived from condensation of ketoacyl subunits); and sterol lipids and prenol lipids (derived from condensation of isoprene subunits).
Although the term lipid is sometimes used as a synonym for fats, fats are a subgroup of lipids called triglycerides. Lipids also encompass molecules such as fatty acids and their derivatives (including tri-, di-, and monoglycerides and phospholipids), as well as other sterol-containing metabolites such as cholesterol. Although humans and other mammals use various biosynthetic pathways to both break down and synthesize lipids, some essential lipids cannot be made this way and must be obtained from the diet.
Categories of lipids
Fatty acyls
Fatty acyls, a generic term for describing fatty acids, their conjugates and derivatives, are a diverse group of molecules synthesized by chain-elongation of an acetyl-CoA primer with malonyl-CoA or methylmalonyl-CoA groups in a process called fatty acid synthesis. They are made of a hydrocarbon chain that terminates with a carboxylic acid group; this arrangement confers the molecule with a polar, hydrophilic end, and a nonpolar, hydrophobic end that is insoluble in water. The fatty acid structure is one of the most fundamental categories of biological lipids, and is commonly used as a building block of more structurally complex lipids. The carbon chain, typically between four to 24 carbons long, may be saturated or unsaturated, and may be attached to functional groups containing oxygen, halogens, nitrogen and sulfur. Where a double bond exists, there is the possibility of either a cis or trans geometric isomerism, which significantly affects the molecule's molecular configuration. Cis-double bounds cause the fatty acid chain to bend, an effect that is more pronounced the more double bonds there are in a chain. This in turn plays an important role in the structure and function of cell membranes. Most naturally occurring fatty acids are of the cis configuration, although the trans form does exist in some natural and partially hydrogenated fats and oils.
Examples of biologically important fatty acids are the eicosanoids, derived primarily from arachidonic acid and eicosapentaenoic acid, which include prostaglandins, leukotrienes, and thromboxanes. Other major lipid classes in the fatty acid category are the fatty esters and fatty amides. Fatty esters include important biochemical intermediates such as wax esters, fatty acid thioester coenzyme A derivatives, fatty acid thioester ACP derivatives and fatty acid carnitines. The fatty amides include N-acyl ethanolamines, such as the cannabinoid neurotransmitter anandamide.
Glycerolipids
Glycerolipids are composed mainly of mono-, di- and tri-substituted glycerols, the most well-known being the fatty acid esters of glycerol (triacylglycerols), also known as triglycerides. In these compounds, the three hydroxyl groups of glycerol are each esterified, usually by different fatty acids. Because they function as a food store, these lipids comprise the bulk of storage fat in animal tissues. The hydrolysis of the ester bonds of triacylglycerols and the release of glycerol and fatty acids from adipose tissue is called fat mobilization.
Additional subclasses of glycerolipids are represented by glycosylglycerols, which are characterized by the presence of one or more sugar residues attached to glycerol via a glycosidic linkage. Examples of structures in this category are the digalactosyldiacylglycerols found in plant membranes and seminolipid from mammalian sperm cells.
Glycerophospholipids
Glycerophospholipids, also referred to as phospholipids, are ubiquitous in nature and are key components of the lipid bilayer of cells, as well as being involved in metabolism and cell signaling. Neural tissue (including the brain) contains relatively high amounts of glycerophospholipids, and alterations in their composition has been implicated in various neurological disorders. Glycerophospholipids may be subdivided into distinct classes, based on the nature of the polar headgroup at the sn-3 position of the glycerol backbone in eukaryotes and eubacteria, or the sn-1 position in the case of archaebacteria.
Phosphatidylethanolamine (Examples of glycerophospholipids found in biological membranes are phosphatidylcholine (also known as PC, GPCho or lecithin), phosphatidylethanolamine (PE or GPEtn) and phosphatidylserine (PS or GPSer). In addition to serving as a primary component of cellular membranes and binding sites for intra- and intercellular proteins, some glycerophospholipids in eukaryotic cells, such as phosphatidylinositols and phosphatidic acids are either precursors of, or are themselves, membrane-derived second messengers. Typically, one or both of these hydroxyl groups are acylated with long-chain fatty acids, but there are also alkyl-linked and 1Z-alkenyl-linked (plasmalogen) glycerophospholipids, as well as dialkylether variants in archaebacteria.
Sphingolipids
Sphingolipids are a complex family of compounds that share a common structural feature, a sphingoid base backbone that is synthesized de novo from the amino acid serine and a long-chain fatty acyl CoA, then converted into ceramides, phosphosphingolipids, glycosphingolipids and other compounds. The major sphingoid base of mammals is commonly referred to as sphingosine. Ceramides (N-acyl-sphingoid bases) are a major subclass of sphingoid base derivatives with an amide-linked fatty acid. The fatty acids are typically saturated or mono-unsaturated with chain lengths from 16 to 26 carbon atoms.
Sphingomyelin.The major phosphosphingolipids of mammals are sphingomyelins (ceramide phosphocholines), whereas insects contain mainly ceramide phosphoethanolamines and fungi have phytoceramide phosphoinositols and mannose-containing headgroups. The glycosphingolipids are a diverse family of molecules composed of one or more sugar residues linked via a glycosidic bond to the sphingoid base. Examples of these are the simple and complex glycosphingolipids such as cerebrosides and gangliosides.
Sterol lipids
Sterol lipids, such as cholesterol and its derivatives, are an important component of membrane lipids, along with the glycerophospholipids and sphingomyelins. The steroids, all derived from the same fused four-ring core structure, have different biological roles as hormones and signaling molecules. The eighteen-carbon (C18) steroids include the estrogen family whereas the C19 steroids comprise the androgens such as testosterone and androsterone. The C21 subclass includes the progestogens as well as the glucocorticoids and mineralocorticoids. The secosteroids, comprising various forms of vitamin D, are characterized by cleavage of the B ring of the core structure. Other examples of sterols are the bile acids and their conjugates, which in mammals are oxidized derivatives of cholesterol and are synthesized in the liver. The plant equivalents are the phytosterols, such as β-sitosterol, stigmasterol, and brassicasterol; the latter compound is also used as a biomarker for algal growth. The predominant sterol in fungal cell membranes is ergosterol.
Prenol lipids
Prenol lipids are synthesized from the 5-carbon precursors isopentenyl diphosphate and dimethylallyl diphosphate that are produced mainly via the mevalonic acid (MVA) pathway. The simple isoprenoids (linear alcohols, diphosphates, etc.) are formed by the successive addition of C5 units, and are classified according to number of these terpene units. Structures containing greater than 40 carbons are known as polyterpenes. Carotenoids are important simple isoprenoids that function as antioxidants and as precursors of vitamin A. Another biologically important class of molecules is exemplified by the quinones and hydroquinones, which contain an isoprenoid tail attached to a quinonoid core of non-isoprenoid origin.Vitamin E and vitamin K, as well as the ubiquinones, are examples of this class. Prokaryotes synthesize polyprenols (called bactoprenols) in which the terminal isoprenoid unit attached to oxygen remains unsaturated, whereas in animal polyprenols (dolichols) the terminal isoprenoid is reduced.
Saccharolipids
Structure of the saccharolipid Kdo2-Lipid A. Glucosamine residues in blue, Kdo residues in red, acyl chains in black and phosphate groups in green.Saccharolipids describe compounds in which fatty acids are linked directly to a sugar backbone, forming structures that are compatible with membrane bilayers. In the saccharolipids, a monosaccharide substitutes for the glycerol backbone present in glycerolipids and glycerophospholipids. The most familiar saccharolipids are the acylated glucosamine precursors of the Lipid A component of the lipopolysaccharides in Gram-negative bacteria. Typical lipid A molecules are disaccharides of glucosamine, which are derivatized with as many as seven fatty-acyl chains. The minimal lipopolysaccharide required for growth in E. coli is Kdo2-Lipid A, a hexa-acylated disaccharide of glucosamine that is glycosylated with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues.
Polyketides
Polyketides are synthesized by polymerization of acetyl and propionyl subunits by classic enzymes as well as iterative and multimodular enzymes that share mechanistic features with the fatty acid synthases. They comprise a large number of secondary metabolites and natural products from animal, plant, bacterial, fungal and marine sources, and have great structural diversity. Many polyketides are cyclic molecules whose backbones are often further modified by glycosylation, methylation, hydroxylation, oxidation, and/or other processes. Many commonly used anti-microbial, anti-parasitic, and anti-cancer agents are polyketides or polyketide derivatives, such as erythromycins, tetracyclines, avermectins, and antitumor epothilones.
Biological functions
Membranes
Eukaryotic cells are compartmentalized into membrane-bound organelles which carry out different biological functions. The glycerophospholipids are the main structural component of biological membranes, such as the cellular plasma membrane and the intracellular membranes of organelles; in animal cells the plasma membrane physically separates the intracellular components from the extracellular environment. The glycerophospholipids are amphipathic molecules (containing both hydrophobic and hydrophilic regions) that contain a glycerol core linked to two fatty acid-derived "tails" by ester linkages and to one "head" group by a phosphate ester linkage. While glycerophospholipids are the major component of biological membranes, other non-glyceride lipid components such as sphingomyelin and sterols (mainly cholesterol in animal cell membranes) are also found in biological membranes.In plants and algae, the galactosyldiacylglycerols, and sulfoquinovosyldiacylglycerol,which lack a phosphate group, are important components of membranes of chloroplasts and related organelles and are the most abundant lipids in photosynthetic tissues, including those of higher plants, algae and certain bacteria.
Bilayers have been found to exhibit high levels of birefringence which can be used to probe the degree of order (or disruption) within the bilayer using techniques such as dual polarisation interferometry
Self-organization of phospholipids: a spherical liposome, a micelle and a lipid bilayer.A biological membrane is a form of lipid bilayer. The formation of lipid bilayers is an energetically-preferred process when the glycerophospholipids described above are in an aqueous environment. In an aqueous system, the polar heads of lipids align towards the polar, aqueous environment, while the hydrophobic tails minimize their contact with water and tend to cluster together, forming a vesicle; depending on the concentration of the lipid, this biophysical interaction may result in the formation of micelles, liposomes, or lipid bilayers. Other aggregations are also observed and form part of the polymorphism of amphiphile (lipid) behavior. Phase behavior is an area of study within biophysics and is the subject of current academic research. Micelles and bilayers form in the polar medium by a process known as the hydrophobic effect. When dissolving a lipophilic or amphiphilic substance in a polar environment, the polar molecules (i.e., water in an aqueous solution) become more ordered around the dissolved lipophilic substance, since the polar molecules cannot form hydrogen bonds to the lipophilic areas of the amphiphile. So in an aqueous environment, the water molecules form an ordered "clathrate" cage around the dissolved lipophilic molecule.
Energy storage
Triacylglycerols, stored in adipose tissue, are a major form of energy storage in animals. The adipocyte, or fat cell, is designed for continuous synthesis and breakdown of triacylglycerols, with breakdown controlled mainly by the activation of hormone-sensitive enzyme lipase.[46] The complete oxidation of fatty acids provides high caloric content, about 9 kcal/g, compared with 4 kcal/g for the breakdown of carbohydrates and proteins. Migratory birds that must fly long distances without eating use stored energy of triacylglycerols to fuel their flights.[47]
Signaling
In recent years, evidence has emerged showing that lipid signaling is a vital part of the cell signaling. Lipid signaling may occur via activation of G protein-coupled or nuclear receptors, and members of several different lipid categories have been identified as signaling molecules and cellular messengers. These include sphingosine-1-phosphate, a sphingolipid derived from ceramide that is a potent messenger molecule involved in regulating calcium mobilization, cell growth, and apoptosis; diacylglycerol (DAG) and the phosphatidylinositol phosphates (PIPs), involved in calcium-mediated activation of protein kinase C; the prostaglandins, which are one type of fatty-acid derived eicosanoid involved in inflammation and immunity; the steroid hormones such as estrogen, testosterone and cortisol, which modulate a host of functions such as reproduction, metabolism and blood pressure; and the oxysterols such as 25-hydroxy-cholesterol that are liver X receptor agonists.
Other functions
The "fat-soluble" vitamins (A, D, E and K) – which are isoprene-based lipids – are essential nutrients stored in the liver and fatty tissues, with a diverse range of functions. Acyl-carnitines are involved in the transport and metabolism of fatty acids in and out of mitochondria, where they undergo beta oxidation. Polyprenols and their phosphorylated derivatives also play important transport roles, in this case the transport of oligosaccharides across membranes. Polyprenol phosphate sugars and polyprenol diphosphate sugars function in extra-cytoplasmic glycosylation reactions, in extracellular polysaccharide biosynthesis (for instance, peptidoglycan polymerization in bacteria), and in eukaryotic protein N-glycosylation. Cardiolipins are a subclass of glycerophospholipids containing four acyl chains and three glycerol groups that are particularly abundant in the inner mitochondrial membrane.They are believed to activate enzymes involved with oxidative phosphorylation.
Metabolism
The major dietary lipids for humans and other animals are animal and plant triglycerides, sterols, and membrane phospholipids. The process of lipid metabolism synthesizes and degrades the lipid stores and produces the structural and functional lipids characteristic of individual tissues.
Biosynthesis
In animals, when there is an oversupply of dietary carbohydrate, the excess carbohydrate is converted to triacylglycerol. This involves the synthesis of fatty acids from acetyl-CoA and the esterification of fatty acids in the production of triacylglycerol, a process called lipogenesis.Fatty acids are made by fatty acid synthases that polymerize and then reduce acetyl-CoA units. The acyl chains in the fatty acids are extended by a cycle of reactions that add the acetyl group, reduce it to an alcohol, dehydrate it to an alkene group and then reduce it again to an alkane group. The enzymes of fatty acid biosynthesis are divided into two groups, in animals and fungi all these fatty acid synthase reactions are carried out by a single multifunctional protein,while in plant plastids and bacteria separate enzymes perform each step in the pathway. The fatty acids may be subsequently converted to triacylglycerols that are packaged in lipoproteins and secreted from the liver.
The synthesis of unsaturated fatty acids involves a desaturation reaction, whereby a double bond is introduced into the fatty acyl chain. For example, in humans, the desaturation of stearic acid by stearoyl-CoA desaturase-1 produces oleic acid. The doubly-unsaturated fatty acid linoleic acid as well as the triply-unsaturated linolenic acid cannot be synthesized in mammalian tissues, and are therefore essential fatty acids and must be obtained from the diet.
Triacylglycerol synthesis takes place in the endoplasmic reticulum by metabolic pathways in which acyl groups in fatty acyl-CoAs are transferred to the hydroxyl groups of glycerol-3-phosphate and diacylglycerol.
Terpenes and isoprenoids, including the carotenoids, are made by the assembly and modification of isoprene units donated from the reactive precursors isopentenyl pyrophosphate and dimethylallyl pyrophosphate. These precursors can be made in different ways. In animals and archaea, the mevalonate pathway produces these compounds from acetyl-CoA,while in plants and bacteria the non-mevalonate pathway uses pyruvate and glyceraldehyde 3-phosphate as substrates. One important reaction that uses these activated isoprene donors is steroid biosynthesis. Here, the isoprene units are joined together to make squalene and then folded up and formed into a set of rings to make lanosterol. Lanosterol can then be converted into other steroids such as cholesterol and ergosterol.
Degradation
Beta oxidation is the metabolic process by which fatty acids are broken down in the mitochondria and/or in peroxisomes to generate acetyl-CoA. For the most part, fatty acids are oxidized by a mechanism that is similar to, but not identical with, a reversal of the process of fatty acid synthesis. That is, two-carbon fragments are removed sequentially from the carboxyl end of the acid after steps of dehydrogenation, hydration, and oxidation to form a beta-keto acid, which is split by thiolysis. The acetyl-CoA is then ultimately converted into ATP, CO2, and H2O using the citric acid cycle and the electron transport chain. The energy yield of the complete oxidation of the fatty acid palmitate is 106 ATP. Unsaturated and odd-chain fatty acids require additional enzymatic steps for degradation.
Nutrition and health
Most of the lipid found in food is in the form of triacylglycerols, cholesterol and phospholipids. A minimum amount of dietary fat is necessary to facilitate absorption of fat-soluble vitamins (A, D, E and K) and carotenoids.[72] Humans and other mammals have a dietary requirement for certain essential fatty acids, such as linoleic acid (an omega-6 fatty acid) and alpha-linolenic acid (an omega-3 fatty acid) because they cannot be synthesized from simple precursors in the diet.Both of these fatty acids are 18-carbon polyunsaturated fatty acids differing in the number and position of the double bonds. Most vegetable oils are rich in linoleic acid (safflower, sunflower, and corn oils). Alpha-linolenic acid is found in the green leaves of plants, and in selected seeds, nuts and legumes (particularly flax, rapeseed, walnut and soy).[74] Fish oils are particularly rich in the longer-chain omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). A large number of studies have shown positive health benefits associated with consumption of omega-3 fatty acids on infant development, cancer, cardiovascular diseases, and various mental illnesses, such as depression, attention-deficit hyperactivity disorder, and dementia.In contrast, it is now well-established that consumption of trans fats, such as those present in partially hydrogenated vegetable oils, are a risk factor for cardiovascular disease.
A few studies have suggested that total diary fat intake is linked to an increased risk of obesity and diabetes.However, a number of very large studies, including the Women's Health Initiative Dietary Modification Trial, an eight year study of 49,000 women, the Nurses' Health Study and the Health Professionals Follow-up Study, revealed no such links.None of these studies suggested any connection between percentage of calories from fat and risk of cancer, heart disease or weight gain. The Nutrition Source, a website maintained by the Department of Nutrition at the Harvard School of Public Health, summarizes the current evidence on the impact of dietary fat: "Detailed research—much of it done at Harvard—shows that the total amount of fat in the diet isn't really linked with weight or disease."
Organic compound
An organic compound is any member of a large class of chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of compounds such as carbonates, simple oxides of carbon and cyanides, as well as the allotropes of carbon, are considered inorganic. The division between "organic" and "inorganic" carbon compounds while "useful in organizing the vast subject of chemistry...is somewhat arbitrary".
Organic chemistry is the science concerned with all aspects of organic compounds. Organic synthesis is the methodology of their preparation.
Classification
See Organic chemistry#Classification of organic substances
Organic compounds may be classified in a variety of ways. One major distinction is between natural and synthetic compounds. They may also be distinguished by the presence of additional atoms of further elements, so-called heteroatoms. Organometallic compounds constitute a further subsection, characterized by covalent bonds between organic carbon and a metal.
There is also a large number of inorganic carbon compounds to distinguish from organic compounds.
Another distinction, based upon the size of organic compounds, distinguishes between small molecules and polymers.
Natural compounds
Natural compounds refer to those that are produced by plants or animals. Many of these are still extracted from natural sources because they would be far too expensive to be produced artificially. Examples include most sugars, some alkaloids and terpenoids, certain nutrients such as vitamin B12, and in general, those natural products with large or stereoisometrically complicated molecules which are present in reasonable concentrations in living organisms.
Further compounds of prime importance in biochemistry are antigens, carbohydrates, enzymes, hormones, lipids and fatty acids, neurotransmitters, nucleic acids, proteins, peptides and amino acids, vitamins and fats and oils.
Synthetic compounds
Compounds that are prepared by reaction of other compounds are referred to as "synthetic". They may be either compounds that already are found in plants or animals, or those that do not occur naturally.
Many polymers, including all plastics, are organic compounds.
Carbohydrates
[α] or saccharides[β] are the most abundant of the four major classes of biomolecules. They fill numerous roles in living things, such as the storage and transport of energy (e.g., starch, glycogen) and structural components (e.g., cellulose in plants and chitin in animals). In addition, carbohydrates and their derivatives play major roles in the working process of the immune system, fertilization, pathogenesis, blood clotting, and development.[1]
Carbohydrates make up most of the organic matter on Earth because of their extensive roles in all forms of life. First, carbohydrates serve as energy stores, fuels, and metabolic intermediates. Second, ribose and deoxyribose sugars form part of the structural framework of RNA and DNA. Third, polysaccharides are structural elements in the cell walls of bacteria and plants. In fact, cellulose, the main constituent of plant cell walls, is one of the most abundant organic compounds in the biosphere. Fourth, carbohydrates are linked to many proteins and lipids, where they play key roles in mediating interactions between cells and interactions between cells and other elements in the cellular environment.
Carbohydrates are simple organic compounds that are aldehydes or ketones with many hydroxyl groups added, usually one on each carbon atom that is not part of the aldehyde or ketone functional group. The basic carbohydrate units are called monosaccharides; examples are glucose, galactose, and fructose. The general stoichiometric formula of an unmodified monosaccharide is (C·H2O)n, where n is any number of three or greater; however, not all carbohydrates conform to this precise stoichiometric definition (e.g., uronic acids, deoxy-sugars such as fucose), nor are all chemicals that do conform to this definition automatically classified as carbohydrates.
1. Monosaccharides
This can be linked together into what are called polysaccharides (or oligosaccharides) in a large variety of ways. Many carbohydrates contain one or more modified monosaccharide units that have had one or more groups replaced or removed. For example, deoxyribose, a component of DNA, is a modified version of ribose; chitin is composed of repeating units of N-acetylglucosamine, a nitrogen-containing form of glucose.
While the scientific nomenclature of carbohydrates is complex, the names of carbohydrates very often end in the suffix -ose. Glycoinformatics is the specialised field of study that deals with the specific and unique bioinformatics of carbohydrates.
2. Dissacharide
Two joined monosaccharides are called a disaccharide and these are the simplest polysaccharides. Examples include sucrose and lactose. They are composed of two monosaccharide units bound together by a covalent bond known as a glycosidic linkage formed via a dehydration reaction, resulting in the loss of a hydrogen atom from one monosaccharide and a hydroxyl group from the other. The formula of unmodified disaccharides is C12H22O11. Although there are numerous kinds of disaccharides, a handful of disaccharides are particularly notable.
Sucrose, pictured to the right, is the most abundant disaccharide, and the main form in which carbohydrates are transported in plants. It is composed of one D-glucose molecule and one D-fructose molecule. The systematic name for sucrose, O-α-D-glucopyranosyl-(1→2)-D-fructofuranoside, indicates four things:
Its monosaccharides: glucose and fructose
Their ring types: glucose is a pyranose, and fructose is a furanose
How they are linked together: the oxygen on carbon number 1 (C1) of α-D-glucose is linked to the C2 of D-fructose.
The -oside suffix indicates that the anomeric carbon of both monosaccharides participates in the glycosidic bond.
Lactose, a disaccharide composed of one D-galactose molecule and one D-glucose molecule, occurs naturally in mammalian milk. The systematic name for lactose is O-β-D-galactopyranosyl-(1→4)-D-glucopyranose. Other notable disaccharides include maltose (two D-glucoses linked α-1,4) and cellulobiose (two D-glucoses linked β-1,4).
3. Polysaccharide
Oligosaccharides and polysaccharides are composed of longer chains of monosaccharide units bound together by glycosidic bonds. The distinction between the two is based upon the number of monosaccharide units present in the chain. Oligosaccharides typically contain between two and nine monosaccharide units, and polysaccharides contain greater than ten monosaccharide units. Definitions of how large a carbohydrate must be to fall into each category vary according to personal opinion. Examples of oligosaccharides include the disaccharides mentioned above, the trisaccharide raffinose and the tetrasaccharide stachyose.
Oligosaccharides are found as a common form of protein posttranslational modification. Such posttranslational modifications include the Lewis and ABO oligosaccharides responsible for blood group classifications and so of tissue incompatibilities, the alpha-Gal epitope responsible for hyperacute rejection in xenotransplanation, and O-GlcNAc modifications.
Polysaccharides represent an important class of biological polymers. Their function in living organisms is usually either structure- or storage-related. Starch (a polymer of glucose) is used as a storage polysaccharide in plants, being found in the form of both amylose and the branched amylopectin. In animals, the structurally-similar glucose polymer is the more densely-branched glycogen, sometimes called 'animal starch'. Glycogen's properties allow it to be metabolized more quickly, which suits the active lives of moving animals.
Cellulose and chitin are examples of structural polysaccharides. Cellulose is used in the cell walls of plants and other organisms, and is claimed to be the most abundant organic molecule on earth. It has many uses such as a significant role in the paper and textile industries, and is used as a feedstock for the production of rayon (via the viscose process), cellulose acetate, celluloid, and nitrocellulose. Chitin has a similar structure, but has nitrogen-containing side branches, increasing its strength. It is found in arthropod exoskeletons and in the cell walls of some fungi. It also has multiple uses, including surgical threads.
Other polysaccharides include callose or
laminarin, chrysolaminarin, xylan, mannan, fucoidan,starch and galactomannan.
Nutrition
Grain products: rich sources of complex and simple carbohydratesFoods high in carbohydrates include breads, pastas, beans, potatoes, bran, rice, and cereals. Most such foods are high in starch. Carbohydrates require less water to digest than proteins or fats and are the most common source of energy in living things. Proteins and fat are necessary building components for body tissue and cells, and are also a source of energy for most organisms.
Carbohydrates are not essential nutrients in humans: the body can obtain all its energy from protein and fats. However, the brain and neurons generally cannot burn fat and need glucose for energy; the body can make some glucose from a few of the amino acids in protein and also from the glycerol backbone in triglycerides. Carbohydrate contains 15.8 kilojoules (3.75 calories) and proteins 16.8 kilojoules (4 calories) per gram, respectively, while fats contain 37.8 kilojoules (9 calories) per gram. In the case of protein, this is somewhat misleading as only some amino acids are usable for fuel. Likewise, in humans, only some carbohydrates are usable for fuel, as in many monosaccharides and some disaccharides. Other carbohydrate types can be used, but only with the assistance of gut bacteria. Ruminants and termites can even process cellulose, which is indigestible to other organisms.
Based on the effects on risk of heart disease and obesity, the Institute of Medicine recommends that American and Canadian adults get between 40-65% of dietary energy from carbohydrates.The Food and Agriculture Organization and World Health Organization jointly recommend that national dietary guidelines set a goal of 55-75% of total energy from carbohydrates, but only 10% directly from sugars (their term for simple carbohydrates).
Classification
Carbohydrates can be classified as simple (monosaccharides and disaccharides) or complex (oligosaccharides and polysaccharides). The term complex carbohydrate was first used in the Senate Select Committee publication Dietary Goals for the United States (1977), where it denoted "fruit, vegetables and whole-grains".[9] Dietary guidelines generally recommend that complex carbohydrates, and such nutrient-rich simple carbohydrate sources such as fruit (glucose or fructose) and dairy products (lactose) make up the bulk of carbohydrate consumption. This excludes such sources of simple sugars as candy and sugary drinks.
The USDA's Dietary Guidelines for Americans 2005 dispensed with the simple/complex distinction, instead recommending fiber-rich foods and whole grains.[10]
The glycemic index and glycemic load concepts have been developed to characterize food behavior during human digestion. They rank carbohydrate-rich foods based on the rapidity of their effect on blood glucose levels. The insulin index is a similar, more recent classification method that ranks foods based on their effects on blood insulin levels, which are caused by glucose (or starch) and some amino acids in food. Glycemic index is a measure of how quickly food glucose is absorbed, while glycemic load is a measure of the total absorbable glucose in foods.

Saturday, August 15, 2009
Microscope
A microscope (from the Greek: μικρός, mikrós, "small" and σκοπεῖν, skopeîn, "to look" or "see") is an instrument for viewing objects that are too small to be seen by the naked or unaided eye. The science of investigating small objects using such an instrument is called microscopy. The term microscopic means minute or very small, not visible with the eye unless aided by a microscope. Anton Van Leeuwenhoek's new, improved microscope allowed people to see things no human had ever seen before.
History
The first true microscope was made around 1595 in Middelburg, The Netherlands.[1] Three different eyeglass makers have been given credit for the invention: Hans Lippershey (who also developed the first real telescope); Sacharias Jansen; and his son, Zacharias. The coining of the name "microscope" has been credited to Giovanni Faber, who gave that name to Galileo Galilei's compound microscope in 1625.[2] (Galileo had called it the "occhiolino" or "little eye".)
The most common type of microscope—and the first to be invented—is the optical microscope. This is an optical instrument containing one or more lenses that produce an enlarged image of an object placed in the focal plane of the lens(es). There are, however, many other microscope designs.

History
The first true microscope was made around 1595 in Middelburg, The Netherlands.[1] Three different eyeglass makers have been given credit for the invention: Hans Lippershey (who also developed the first real telescope); Sacharias Jansen; and his son, Zacharias. The coining of the name "microscope" has been credited to Giovanni Faber, who gave that name to Galileo Galilei's compound microscope in 1625.[2] (Galileo had called it the "occhiolino" or "little eye".)
The most common type of microscope—and the first to be invented—is the optical microscope. This is an optical instrument containing one or more lenses that produce an enlarged image of an object placed in the focal plane of the lens(es). There are, however, many other microscope designs.

Meiosis
In biology, meiosis (pronounced /maɪˈoʊsɨs/) is a process of reductional division in which the number of chromosomes per cell is halved. In animals, meiosis always results in the formation of gametes, while in other organisms it can give rise to spores. As with mitosis, before meiosis begins, the DNA in the original cell is replicated during S-phase of the cell cycle. Two cell divisions separate the replicated chromosomes into four haploid gametes or spores.
Meiosis is essential for sexual reproduction and therefore occurs in all eukaryotes (including single-celled organisms) that reproduce sexually. A few eukaryotes, notably the Bdelloid rotifers, have lost the ability to carry out meiosis and have acquired the ability to reproduce by parthenogenesis. Meiosis does not occur in archaea or bacteria, which reproduce via asexual processes such as binary fission.
During meiosis, the genome of a diploid germ cell, which is composed of long segments of DNA packaged into chromosomes, undergoes DNA replication followed by two rounds of division, resulting in four haploid cells. Each of these cells contain one complete set of chromosomes, or half of the genetic content of the original cell. If meiosis produces gametes, these cells must fuse during fertilization to create a new diploid cell, or zygote before any new growth can occur. Thus, the division mechanism of meiosis is a reciprocal process to the joining of two genomes that occurs at fertilization. Because the chromosomes of each parent undergo homologous recombination during meiosis, each gamete, and thus each zygote, will have a unique genetic blueprint encoded in its DNA. Together, meiosis and fertilization constitute sexuality in the eukaryotes, and generate genetically distinct individuals in populations.
In all plants, and in many protists, meiosis results in the formation of haploid cells that can divide vegetatively without undergoing fertilization, referred to as spores. In these groups, gametes are produced by mitosis.
Meiosis uses many of the same biochemical mechanisms employed during mitosis to accomplish the redistribution of chromosomes. There are several features unique to meiosis, most importantly the pairing and recombination between homologous chromosomes.
Meiosis comes from the root -meio, meaning less.
History
Meiosis was discovered and described for the first time in sea urchin eggs in 1876, by noted German biologist Oscar Hertwig (1849-1922). It was described again in 1883, at the level of chromosomes, by Belgian zoologist Edouard Van Beneden (1846-1910), in Ascaris worms' eggs. The significance of meiosis for reproduction and inheritance, however, was described only in 1890 by German biologist August Weismann (1834-1914), who noted that two cell divisions were necessary to transform one diploid cell into four haploid cells if the number of chromosomes had to be maintained. In 1911 the American geneticist Thomas Hunt Morgan (1866-1945) observed crossover in Drosophila melanogaster meiosis and provided the first genetic evidence that genes are transmitted on chromosomes.
Evolution
Meiosis is thought to have appeared 1.4 billion years ago. The only supergroup of eukaryotes which does not have meiosis in all organisms is excavata. The other five major supergroups, opisthokonts, amoebozoa, rhizaria, archaeplastida and chromalveolates all seem to have genes for meiosis universally present, even if not always functional. Some excavata species do have meiosis which is consistent with the hypothesis that this group is an ancient, paraphyletic grade. An example of eukaryotic organism in which meiosis does not exist is euglenoid.
Occurrence of meiosis in eukaryotic life cycles
Meiosis occur in eukaryotic life cycles involving sexual reproduction, comprising of the constant cyclical process of meiosis and fertilization. This takes place alongside normal mitotic cell division. In multicellular organisms, there is an intermediary step between the diploid and haploid transition where the organism grows. The organism will then produce the germ cells that continue in the life cycle. The rest of the cells, called somatic cells, function within the organism and will die with it.
Cycling meiosis and fertilization events produces a series of transitions back and forth between alternating haploid and diploid states. The organism phase of the life cycle can occur either during the diploid state (gametic or diploid life cycle), during the haploid state (zygotic or haploid life cycle), or both (sporic or haplodiploid life cycle, in which there two distinct organism phases, one during the haploid state and the other during the diploid state). In this sense, there are three types of life cycles that utilize sexual reproduction, differentiated by the location of the organisms phase(s).
In the gametic life cycle, of which humans are a part, the species is diploid, grown from a diploid cell called the zygote. The organism's diploid germ-line stem cells undergo meiosis to create haploid gametes (the spermatozoa for males and ova for females), which fertilize to form the zygote. The diploid zygote undergoes repeated cellular division by mitosis to grow into the organism. Mitosis is a related process to meiosis that creates two cells that are genetically identical to the parent cell. The general principle is that mitosis creates somatic cells and meiosis creates germ cells.
In the zygotic life cycle the species is haploid instead, spawned by the proliferation and differentiation of a single haploid cell called the gamete. Two organisms of opposing gender contribute their haploid germ cells to form a diploid zygote. The zygote undergoes meiosis immediately, creating four haploid cells. These cells undergo mitosis to create the organism. Many fungi and many protozoa are members of the zygotic life cycle.
Finally, in the sporic life cycle, the living organism alternates between haploid and diploid states. Consequently, this cycle is also known as the alternation of generations. The diploid organism's germ-line cells undergo meiosis to produce gametes. The gametes proliferate by mitosis, growing into a haploid organism. The haploid organism's germ cells then combine with another haploid organism's cells, creating the zygote. The zygote undergoes repeated mitosis and differentiation to become the diploid organism again. The sporic life cycle can be considered a fusion of the gametic and zygotic life cycles.
Process
Because meiosis is a "one-way" process, it cannot be said to engage in a cell cycle as mitosis does. However, the preparatory steps that lead up to meiosis are identical in pattern and name to the interphase of the mitotic cell cycle.
Interphase is divided into three phases:
Growth 1 (G1) phase: This is a very active period, where the cell synthesizes its vast array of proteins, including the enzymes and structural proteins it will need for growth. In G1 stage each of the chromosomes consists of a single (very long) molecule of DNA. In humans, at this point cells are 46 chromosomes, 2N, identical to somatic cells.
Synthesis (S) phase: The genetic material is replicated: each of its chromosomes duplicates, producing 46 chromosomes each made up of two sister chromatids. The cell is still considered diploid because it still contains the same number of centromeres. The identical sister chromatids have not yet condensed into the densely packaged chromosomes visible with the light microscope. This will take place during prophase I in meiosis.
Growth 2 (G2) phase: G2 phase is absent in Meiosis
Interphase is followed by meiosis I and then meiosis II. Meiosis I consists of separating the pairs of homologous chromosome, each made up of two sister chromatids, into two cells. One entire haploid content of chromosomes is contained in each of the resulting daughter cells; the first meiotic division therefore reduces the ploidy of the original cell by a factor of 2.
Meiosis II consists of decoupling each chromosome's sister strands (chromatids), and segregating the individual chromatids into haploid daughter cells. The two cells resulting from meiosis I divide during meiosis II, creating 4 haploid daughter cells. Meiosis I and II are each divided into prophase, metaphase, anaphase, and telophase stages, similar in purpose to their analogous subphases in the mitotic cell cycle. Therefore, meiosis includes the stages of meiosis I (prophase I, metaphase I, anaphase I, telophase I), and meiosis II (prophase II, metaphase II, anaphase II, telophase II).
Meiosis generates genetic diversity in two ways: (1) independent alignment and subsequent separation of homologous chromosome pairs during the first meiotic division allows a random and independent selection of each chromosome segregates into each gamete; and (2) physical exchange of homologous chromosomal regions by recombination during prophase I results in new combinations of DNA within chromosomes.
Meiosis-phases
Meiosis I
Meiosis I separates homologous chromosomes, producing two haploid cells (23 chromosomes, N in humans), so meiosis I is referred to as a reductional division. A regular diploid human cell contains 46 chromosomes and is considered 2N because it contains 23 pairs of homologous chromosomes. However, after meiosis I, although the cell contains 46 chromatids it is only considered as being N, with 23 chromosomes, because later in anaphase I the sister chromatids will remain together as the spindle pulls the pair toward the pole of the new cell. In meiosis II, an equational division similar to mitosis will occur whereby the sister chromatids are finally split, creating a total of 4 haploid cells (23 chromosomes, N) per daughter cell from the first division.
Prophase I
During prophase I, DNA is exchanged between homologous chromosomes in a process called homologous recombination. This often results in chromosomal crossover. The new combinations of DNA created during crossover are a significant source of genetic variation, and may result in beneficial new combinations of alleles. The paired and replicated chromosomes are called bivalents or tetrads, which have two chromosomes and four chromatids, with one chromosome coming from each parent. At this stage, non-sister chromatids may cross-over at points called chiasmata (plural; singular chiasma).
Leptotene
The first stage of prophase I is the leptotene stage, also known as leptonema, from Greek words meaning "thin threads".During this stage, individual chromosomes begin to condense into long strands within the nucleus. However the two sister chromatids are still so tightly bound that they are indistinguishable from one another.
Zygotene
The zygotene stage, also known as zygonema, from Greek words meaning "paired threads",occurs as the chromosomes approximately line up with each other into homologous chromosomes. This is called the bouquet stage because of the way the telomeres cluster at one end of the nucleus.
Pachytene
The pachytene stage, also known as pachynema, from Greek words meaning "thick threads", contains the following chromosomal crossover. Nonsister chromatids of homologous chromosomes randomly exchange segments of genetic information over regions of homology. (Sex chromosomes, however, are not wholly identical, and only exchange information over a small region of homology.) Exchange takes place at sites where recombination nodules (the aforementioned chiasmata) have formed. The exchange of information between the non-sister chromatids results in a recombination of information; each chromosome has the complete set of information it had before, and there are no gaps formed as a result of the process. Because the chromosomes cannot be distinguished in the synaptonemal complex, the actual act of crossing over is not perceivable through the microscope.
Diplotene
During the diplotene stage, also known as diplonema, from Greek words meaning "two threads",the synaptonemal complex degrades and homologous chromosomes separate from one another a little. The chromosomes themselves uncoil a bit, allowing some transcription of DNA. However, the homologous chromosomes of each bivalent remain tightly bound at chiasmata, the regions where crossing-over occurred. The chiasmata remain on the chromosomes until they are severed in Anaphase I.
In human fetal oogenesis all developing oocytes develop to this stage and stop before birth. This suspended state is referred to as the dictyotene stage and remains so until puberty. In males, only spermatogonia(Spermatogenesis) exist until meiosis begins at puberty.
Diakinesis
Chromosomes condense further during the diakinesis stage, from Greek words meaning "moving through".This is the first point in meiosis where the four parts of the tetrads are actually visible. Sites of crossing over entangle together, effectively overlapping, making chiasmata clearly visible. Other than this observation, the rest of the stage closely resembles prometaphase of mitosis; the nucleoli disappear, the nuclear membrane disintegrates into vesicles, and the meiotic spindle begins to form.
Synchronous processes
During these stages, two centrosomes, containing a pair of centrioles in animal cells, migrate to the two poles of the cell. These centrosomes, which were duplicated during S-phase, function as microtubule organizing centers nucleating microtubules, which are essentially cellular ropes and poles. The microtubules invade the nuclear region after the nuclear envelope disintegrates, attaching to the chromosomes at the kinetochore. The kinetochore functions as a motor, pulling the chromosome along the attached microtubule toward the originating centriole, like a train on a track. There are four kinetochores on each tetrad, but the pair of kinetochores on each sister chromatid fuses and functions as a unit during meiosis I.
Microtubules that attach to the kinetochores are known as kinetochore microtubules. Other microtubules will interact with microtubules from the opposite centriole: these are called nonkinetochore microtubules or polar microtubules. A third type of microtubules, the aster microtubules, radiates from the centrosome into the cytoplasm or contacts components of the membrane skeleton.
Metaphase I
Homologous pairs move together along the metaphase plate: As kinetochore microtubules from both centrioles attach to their respective kinetochores, the homologous chromosomes align along an equatorial plane that bisects the spindle, due to continuous counterbalancing forces exerted on the bivalents by the microtubules emanating from the two kinetochores of homologous chromosomes. The physical basis of the independent assortment of chromosomes is the random orientation of each bivalent along the metaphase plate, with respect to the orientation of the other bivalents along the same equatorial line.
Anaphase I
Kinetochore microtubules shorten, severing the recombination nodules and pulling homologous chromosomes apart. Since each chromosome has only one functional unit of a pair of kinetochores[3], whole chromosomes are pulled toward opposing poles, forming two haploid sets. Each chromosome still contains a pair of sister chromatids. Nonkinetochore microtubules lengthen, pushing the centrioles farther apart. The cell elongates in preparation for division down the center.
Telophase I
The last meiotic division effectively ends when the chromosomes arrive at the poles. Each daughter cell now has half the number of chromosomes but each chromosome consists of a pair of chromatids. The microtubules that make up the spindle network disappear, and a new nuclear membrane surrounds each haploid set. The chromosomes uncoil back into chromatin. Cytokinesis, the pinching of the cell membrane in animal cells or the formation of the cell wall in plant cells, occurs, completing the creation of two daughter cells. Sister chromatids remain attached during telophase I.
Cells may enter a period of rest known as interkinesis or interphase II. No DNA replication occurs during this stage.
Meiosis II
Meiosis II is the second part of the meiotic process. Much of the process is similar to mitosis. The end result is production of four haploid cells (23 chromosomes, 1N in humans) from the two haploid cells (23 chromosomes, 1N * each of the chromosomes consisting of two sister chromatids) produced in meiosis I. The four main steps of Meiosis II are: Prophase II, Metaphase II, Anaphase II, and Telophase II.
Prophase II takes an inversely proportional time compared to telophase I. In this prophase we see the disappearance of the nucleoli and the nuclear envelope again as well as the shortening and thickening of the chromatids. Centrioles move to the polar regions and arrange spindle fibers for the second meiotic division.
In metaphase II, the centromeres contain two kinetochores that attach to spindle fibers from the centrosomes (centrioles) at each pole. The new equatorial metaphase plate is rotated by 90 degrees when compared to meiosis I, perpendicular to the previous plate.
This is followed by anaphase II, where the centromeres are cleaved, allowing microtubules attached to the kinetochores to pull the sister chromatids apart. The sister chromatids by convention are now called sister chromosomes as they move toward opposing poles.
The process ends with telophase II, which is similar to telophase I, and is marked by uncoiling and lengthening of the chromosomes and the disappearance of the spindle. Nuclear envelopes reform and cleavage or cell wall formation eventually produces a total of four daughter cells, each with a haploid set of chromosomes. Meiosis is now complete and ends up with four new daughter cells.
[edit] Significance
Meiosis facilitates stable sexual reproduction. Without the halving of ploidy, or chromosome count, fertilization would result in zygotes that have twice the number of chromosomes as the zygotes from the previous generation. Successive generations would have an exponential increase in chromosome count. In organisms that are normally diploid, polyploidy, the state of having three or more sets of chromosomes, results in extreme developmental abnormalities or lethality [4]. Polyploidy is poorly tolerated in most animal species. Plants, however, regularly produce fertile, viable polyploids. Polyploidy has been implicated as an important mechanism in plant speciation.
Most importantly, recombination and independent assortment of homologous chromosomes allow for a greater diversity of genotypes in the population. This produces genetic variation in gametes that promote genetic and phenotypic variation in a population of offspring.
Nondisjunction
The normal separation of chromosomes in meiosis I or sister chromatids in meiosis II is termed disjunction. When the separation is not normal, it is called nondisjunction. This results in the production of gametes which have either too many of too few of a particular chromosome, and is a common mechanism for trisomy or monosomy. Nondisjunction can occur in the meiosis I or meiosis II, phases of cellular reproduction, or during mitosis.
This is a cause of several medical conditions in humans (such as):
Down Syndrome - trisomy of chromosome 21
Patau Syndrome - trisomy of chromosome 13
Edward Syndrome - trisomy of chromosome 18
Klinefelter Syndrome - extra X chromosomes in males - ie XXY, XXXY, XXXXY
Turner Syndrome - lacking of one X chromosome in females - ie XO
Triple X syndrome - an extra X chromosome in females
XYY Syndrome - an extra Y chromosome in males
[edit] Meiosis in mammals
In females, meiosis occurs in cells known as oogonia (singular: oogonium). Each oogonium that initiates meiosis will divide twice to form a single oocyte and two polar bodies.However, before these divisions occur, these cells stop at the diplotene stage of meiosis I and lay dormant within a protective shell of somatic cells called the follicle. Follicles begin growth at a steady pace in a process known as folliculogenesis, and a small number enter the menstrual cycle. Menstruated oocytes continue meiosis I and arrest at meiosis II until fertilization. The process of meiosis in females occurs during oogenesis, and differs from the typical meiosis in that it features a long period of meiotic arrest known as the Dictyate stage and lacks the assistance of centrosomes.
In males, meiosis occurs in precursor cells known as spermatogonia that divide twice to become sperm. These cells continuously divide without arrest in the seminiferous tubules of the testicles. Sperm is produced at a steady pace. The process of meiosis in males occurs during spermatogenesis.
In female mammals, meiosis begins immediately after primordial germ cells migrate to the ovary in the embryo, but in the males, meiosis begins years later at the time of puberty. It is retinoic acid, derived from the primitive kidney (mesonephros) that stimulates meiosis in ovarian oogonia. Tissues of the male testis suppress meiosis by degrading retinoic acid, a stimulator of meiosis. This is overcome at puberty when cells within seminiferous tubules called Sertoli cells start making their own retinoic acid. Sensitivity to retinoic acid is also adjusted by proteins called nanos and DAZL.
Eukaryotic Cells
Eukaryotic cells (from the Greek meaning truly nuclear) comprise all of the life kingdoms except monera. They can be easily distinguished through a membrane-bound nucleus.

Eukaryotic cells also contain many internal membrane-bound structures called organelles. These organelles such as the mitochondrion or chloroplast serve to perform metabolic functions and energy conversion. Other organelles like intracellular filaments provide structural support and cellular motility. The function of individual organelles is described in detail in the Cell Anatomy.

Another important member of the eukaryote family is the plant cell. They function essentially in the same manner as other eukaryotic cells, but there are three unique structures which set them apart. Plastids, cell walls, and vacuoles are present only in plant cells.

Eukaryotic cells also contain many internal membrane-bound structures called organelles. These organelles such as the mitochondrion or chloroplast serve to perform metabolic functions and energy conversion. Other organelles like intracellular filaments provide structural support and cellular motility. The function of individual organelles is described in detail in the Cell Anatomy.

Another important member of the eukaryote family is the plant cell. They function essentially in the same manner as other eukaryotic cells, but there are three unique structures which set them apart. Plastids, cell walls, and vacuoles are present only in plant cells.
Prokaryotic Cells
Diagram of a prokaryotic cell. Notice the internal organelles are not easily distinguishable.

Cells that lack a membrane-bound nucleus are called prokaryotes (from the Greek meaning before nuclei). These cells have few internal structures that are distinguishable under a microscope. Cells in the monera kingdom such as bacteria and cyanobacteria (also known as blue-green algae) are prokaryotes.
Prokaryotic cells differ significantly from eukaryotic cells. They don't have a membrane-bound nucleus and instead of having chromosomal DNA, their genetic information is in a circular loop called a plasmid. Bacterial cells are very small, roughly the size of an animal mitochondrion (about 1-2µm in diameter and 10 µm long). Prokaryotic cells feature three major shapes: rod shaped, spherical, and spiral. Instead of going through elaborate replication processes like eukaryotes, bacterial cells divide by binary fission.
Bacteria perform many important functions on earth. They serve as decomposers, agents of fermentation, and play an important role in our own digestive system. Also, bacteria are involved in many nutrient cycles such as the nitrogen cycle, which restores nitrate into the soil for plants. Unlike eukaryotic cells that depend on oxygen for their metabolism, prokaryotic cells enjoy a diverse array of metabolic functions. For example, some bacteria use sulfur instead of oxygen in their metabolism.

Cells that lack a membrane-bound nucleus are called prokaryotes (from the Greek meaning before nuclei). These cells have few internal structures that are distinguishable under a microscope. Cells in the monera kingdom such as bacteria and cyanobacteria (also known as blue-green algae) are prokaryotes.
Prokaryotic cells differ significantly from eukaryotic cells. They don't have a membrane-bound nucleus and instead of having chromosomal DNA, their genetic information is in a circular loop called a plasmid. Bacterial cells are very small, roughly the size of an animal mitochondrion (about 1-2µm in diameter and 10 µm long). Prokaryotic cells feature three major shapes: rod shaped, spherical, and spiral. Instead of going through elaborate replication processes like eukaryotes, bacterial cells divide by binary fission.
Bacteria perform many important functions on earth. They serve as decomposers, agents of fermentation, and play an important role in our own digestive system. Also, bacteria are involved in many nutrient cycles such as the nitrogen cycle, which restores nitrate into the soil for plants. Unlike eukaryotic cells that depend on oxygen for their metabolism, prokaryotic cells enjoy a diverse array of metabolic functions. For example, some bacteria use sulfur instead of oxygen in their metabolism.


Centrioles - Centrioles are self-replicating organelles made up of nine bundles of microtubules and are found only in animal cells. They appear to help in organizing cell division, but aren't essential to the process.
Cilia and Flagella - For single-celled eukaryotes, cilia and flagella are essential for the locomotion of individual organisms. In multicellular organisms, cilia function to move fluid or materials past an immobile cell as well as moving a cell or group of cells.
Endoplasmic Reticulum - The endoplasmic reticulum is a network of sacs that manufactures, processes, and transports chemical compounds for use inside and outside of the cell. It is connected to the double-layered nuclear envelope, providing a pipeline between the nucleus and the cytoplasm.
Endosomes and Endocytosis - Endosomes are membrane-bound vesicles, formed via a complex family of processes collectively known as endocytosis, and found in the cytoplasm of virtually every animal cell. The basic mechanism of endocytosis is the reverse of what occurs during exocytosis or cellular secretion. It involves the invagination (folding inward) of a cell's plasma membrane to surround macromolecules or other matter diffusing through the extracellular fluid.
Golgi Apparatus - The Golgi apparatus is the distribution and shipping department for the cell's chemical products. It modifies proteins and fats built in the endoplasmic reticulum and prepares them for export to the outside of the cell.
Intermediate Filaments - Intermediate filaments are a very broad class of fibrous proteins that play an important role as both structural and functional elements of the cytoskeleton. Ranging in size from 8 to 12 nanometers, intermediate filaments function as tension-bearing elements to help maintain cell shape and rigidity.
Lysosomes - The main function of these microbodies is digestion. Lysosomes break down cellular waste products and debris from outside the cell into simple compounds, which are transferred to the cytoplasm as new cell-building materials.
Microfilaments - Microfilaments are solid rods made of globular proteins called actin. These filaments are primarily structural in function and are an important component of the cytoskeleton.
Microtubules - These straight, hollow cylinders are found throughout the cytoplasm of all eukaryotic cells (prokaryotes don't have them) and carry out a variety of functions, ranging from transport to structural support.
Mitochondria - Mitochondria are oblong shaped organelles that are found in the cytoplasm of every eukaryotic cell. In the animal cell, they are the main power generators, converting oxygen and nutrients into energy.
Nucleus - The nucleus is a highly specialized organelle that serves as the information processing and administrative center of the cell. This organelle has two major functions: it stores the cell's hereditary material, or DNA, and it coordinates the cell's activities, which include growth, intermediary metabolism, protein synthesis, and reproduction (cell division).
Peroxisomes - Microbodies are a diverse group of organelles that are found in the cytoplasm, roughly spherical and bound by a single membrane. There are several types of microbodies but peroxisomes are the most common.
Plasma Membrane - All living cells have a plasma membrane that encloses their contents. In prokaryotes, the membrane is the inner layer of protection surrounded by a rigid cell wall. Eukaryotic animal cells have only the membrane to contain and protect their contents. These membranes also regulate the passage of molecules in and out of the cells.
Ribosomes - All living cells contain ribosomes, tiny organelles composed of approximately 60 percent RNA and 40 percent protein. In eukaryotes, ribosomes are made of four strands of RNA. In prokaryotes, they consist of three strands of RNA.
Plant cell

Plant cells are eukaryotic cells that differ in several key respects from the cells of other eukaryotic organisms. Their distinctive features include:
A large central vacuole, a water-filled volume enclosed by a membrane known as the tonoplast[1][2] maintains the cell's turgor, controls movement of molecules between the cytosol and sap, stores useful material and digests waste proteins and organelles.
A cell wall composed of cellulose and hemicellulose, pectin and in many cases lignin, and secreted by the protoplast on the outside of the cell membrane. This contrasts with the cell walls of fungi (which are made of chitin), and of bacteria, which are made of peptidoglycan.
Specialised cell-cell communication pathways known as plasmodesmata[3], pores in the primary cell wall through which the plasmalemma and endoplasmic reticulum[4] of adjacent cells are continuous.
Plastids, notably the chloroplasts which contain chlorophyll and the biochemical systems for light harvesting and photosynthesis, but also amyloplasts specialized for starch storage, elaioplasts specialized for fat storage and chromoplasts specialized for synthesis and storage of pigments. As in mitochondria, which have a genome encoding 37 genes[5] plastids have their own genomes of about 100-120 unique genes[6] and probably arose as prokaryotic endosymbionts living in the cells of an early eukaryotic ancestor of the land plants and algae.[7]
Cell division by construction of a phragmoplast as a template for building a cell plate late in cytokinesis is characteristic of land plants and a few groups of algae, notably the Charophytes[8] and the Order Trentepohliales[9]
The sperm of bryophytes have flagellae similar to those in animals,[10][11] but higher plants, (including Gymnosperms and flowering plants) lack the flagellae and centrioles[12] that are present in animal cells.
Characteristics of Life
Before moving on to the development of microscopic organisms on Earth, we must first describe the characteristics of life. All matter, both living and non-living, is composed of miniature chemical building blocks called atoms?. Your body contains billions of hydrogen, oxygen, nitrogen, phosphorus and carbon atoms. The computer you are looking at is also composed of billions of various atoms. However, there is a very important distinction between yourself and your computer - you are alive.
What is life? What does it mean do be alive? How is something made “living”? These are all pertinent questions when discussing the origin of life. Scientists have identified seven basic characteristics of life. For something to be described as living, that something must display all seven of these characteristics. Although many different people have many different opinions about what "living" means, the following characteristics were designated "characteristics of living things" with the consensus of the scientific community.
All living things...
...Are Composed of Cells
Cells? are the basic components of all living things. Some organisms are single celled, like bacteria, or multi-celled, like humans.
This is a single-celled organism called an amoeba. Courtesy The Cell
...Require Energy
Living organisms require energy, usually in the form of ATP?. They use this energy to carry out energy-requiring activities such as metabolism? and locomotion.
This is a molecular model of ATP, the metabolic "energy currency" of all cells. Courtesy Jacob Halaska
...Reproduce
All living organisms reproduce, either by sexual? or asexual? means.
This is an image of a plant cell undergoing mitosis (metaphase stage). Courtesy: S. D. Clark
...Display Heredity
Living organisms inherit traits from the parent organisms that created them. This is called heredity?.
These are two daughter cells containing identical genetic material. Courtesy: Cornell University
...Respond to the Environment
All living things respond to stimuli? in their environment.
This is an image of a stomata opening in response to light stimulus. Courtesy Eric Kruger
...Maintain Homeostasis
All living things maintain a state of internal balance. This is called homeostasis?.
...Evolve and Adapt
Living organisms adapt to their environment and evolve?.
This is a petri dish containing bacteria that cause tuberculosis. Antibiotics were once effective in combating this bacteria, but unfortunately these organisms have adapted a resistance to these antibiotics. Courtesy The Why Files
Using these characteristics, one can categorize things as living or non-living. Imagine if you were a biologist who had never been to Earth before. You have been sent on a mission to determine whether these things called “trees” are alive. How do trees satisfy the seven characteristics of living things? Now investigate some other things on Earth. Are viruses qualified as living?
This is a very interesting question which has instigated many heated debates in scientific circles. Why are viruses such a big deal? Well, viruses are interesting because they are neither inanimate nor living; a virus is "midway between brute matter and living organism" (Wolfhard Weidel). Because of this ambiguity, it is difficult to define and classify viruses. Although viruses are not composed of cells, they possess all the other characteristics of living things. They replicate, require energy (from living cells), adapt, respond to stimuli and display heredity. Because viruses satisfy six of the seven characteristics of living things, they are on the verge of being classified as a living organism. Perhaps the haziness surrounding our very definition of "living" is at fault for not including viruses as a form of life. As scientist Wolfhard Weidel once said, "nothing brings us so close to the riddle of Life - and to its solution - as viruses".
What is life? What does it mean do be alive? How is something made “living”? These are all pertinent questions when discussing the origin of life. Scientists have identified seven basic characteristics of life. For something to be described as living, that something must display all seven of these characteristics. Although many different people have many different opinions about what "living" means, the following characteristics were designated "characteristics of living things" with the consensus of the scientific community.
All living things...
...Are Composed of Cells
Cells? are the basic components of all living things. Some organisms are single celled, like bacteria, or multi-celled, like humans.
This is a single-celled organism called an amoeba. Courtesy The Cell
...Require Energy
Living organisms require energy, usually in the form of ATP?. They use this energy to carry out energy-requiring activities such as metabolism? and locomotion.
This is a molecular model of ATP, the metabolic "energy currency" of all cells. Courtesy Jacob Halaska
...Reproduce
All living organisms reproduce, either by sexual? or asexual? means.
This is an image of a plant cell undergoing mitosis (metaphase stage). Courtesy: S. D. Clark
...Display Heredity
Living organisms inherit traits from the parent organisms that created them. This is called heredity?.
These are two daughter cells containing identical genetic material. Courtesy: Cornell University
...Respond to the Environment
All living things respond to stimuli? in their environment.
This is an image of a stomata opening in response to light stimulus. Courtesy Eric Kruger
...Maintain Homeostasis
All living things maintain a state of internal balance. This is called homeostasis?.
...Evolve and Adapt
Living organisms adapt to their environment and evolve?.
This is a petri dish containing bacteria that cause tuberculosis. Antibiotics were once effective in combating this bacteria, but unfortunately these organisms have adapted a resistance to these antibiotics. Courtesy The Why Files
Using these characteristics, one can categorize things as living or non-living. Imagine if you were a biologist who had never been to Earth before. You have been sent on a mission to determine whether these things called “trees” are alive. How do trees satisfy the seven characteristics of living things? Now investigate some other things on Earth. Are viruses qualified as living?
This is a very interesting question which has instigated many heated debates in scientific circles. Why are viruses such a big deal? Well, viruses are interesting because they are neither inanimate nor living; a virus is "midway between brute matter and living organism" (Wolfhard Weidel). Because of this ambiguity, it is difficult to define and classify viruses. Although viruses are not composed of cells, they possess all the other characteristics of living things. They replicate, require energy (from living cells), adapt, respond to stimuli and display heredity. Because viruses satisfy six of the seven characteristics of living things, they are on the verge of being classified as a living organism. Perhaps the haziness surrounding our very definition of "living" is at fault for not including viruses as a form of life. As scientist Wolfhard Weidel once said, "nothing brings us so close to the riddle of Life - and to its solution - as viruses".
Branches of Biology
Agriculture - study of producing crops from the land, with an emphasis on practical applications
Anatomy - the study of the animal form, with an emphasis on human bodies
Biochemistry - the study of the chemical reactions required for life to exist and function, usually a focus on the cellular level
Bioengineering - the study of biology through the means of engineering with an emphasis on applied knowledge and especially related to biotechnology.
Bioinformatics - also classified as a branch of information technology (IT) it is the study, collection, and storage of genomic data
Biomathematics or Mathematical Biology - the study of biological processes through mathematics, with an emphasis on modeling.
Biomechanics - often considered a branch of medicine, the study of the mechanics of living beings, with an emphasis on applied use through artificial limbs, etc.
Biophysics - the study of biological processes through physics, by applying the theories and methods traditionally used in the physical sciences
Biotechnology - a new and sometimes controversial branch of biology that studies the manipulation of living matter, including genetic modification
Botany - the study of plants
Cell Biology - the study of the cell as a complete unit, and the molecular and chemical interactions that occur within a living cell.
Conservation Biology - the study of the preservation, protection, or restoration of the natural environment, natural ecosystems, vegetation, and wildlife
Cryobiology - the study of the effects of lower than normally preferred temperatures on living beings.
Developmental Biology - the study of the processes through which an organism develops, from zygote to full structure.
Ecology - the study of the ecosystem as a complete unit, with an emphasis on how species and groups of species interact with other living beings and non-living elements.
Entomology - the study of insects
Environmental Biology - the study of the natural world, as a whole or in a particular area, especially as affected by human activity
Epidemiology - a major component of public health research, it is the study of factors affecting the health and illness of populations
Ethology - the study of animal behavior.
Evolution or Evolutionary Biology - the study of the origin and decent of species over time
Genetics - the study of genes and heredity.
Herpetology - the study of reptiles (and amphibians?)
Histology - The study of cells and tissue, a microscopic branch of anatomy.
Ichthyology - the study of fish
Macrobiology - the study of biology on the level of the macroscopic individual (plant, animal, or other living being) as a complete unit.
Mammology - the study of mammals
Marine Biology - the study of ocean ecosystems, plants, animals, and other living beings.
Medicine - the study of the human body in health and disease, with allopathic medicine focusing on alleviating or curing the body from states of disease
Microbiology - the study of microscopic organisms (microorganisms) and their interactions with other living things
Molecular Biology - the study of biology and biological functions at the molecular level, some cross over with biochemistry
Mycology - the study of fungi
Neurobiology - the study of the nervous system, including anatomy, physiology, even pathology
Oceanography - the study of the ocean, including ocean life, environment, geography, weather, and other aspects influencing the ocean. See Marine Biology
Ornithology - the study of birds
Paleontology - the study of fossils and sometimes geographic evidence of prehistoric life
Pathobiology or pathology - the study of diseases, and the causes, processes, nature, and development of disease
Parisitology - the study of parasites and parasitism
Pharmacology - the study and practical application of preparation, use, and effects of drugs and synthetic medicines.
Physiology - the study of the functioning of living organisms and the organs and parts of living organisms
Phytopathology - the study of plant diseases
Pre-medicine - a college major that covers the general aspects of biology as well as specific classes relevant to the study of medicine
Virology - the study of viruses and some other virus-like agents, usually considered part of microbiology or pathology
Zoology - the study of animals and animal life, including classification, physiology, development, and behavior (See also Entomology, Ethology, Herpetology, Ichthyology, Mammology, Ornithology
Anatomy - the study of the animal form, with an emphasis on human bodies
Biochemistry - the study of the chemical reactions required for life to exist and function, usually a focus on the cellular level
Bioengineering - the study of biology through the means of engineering with an emphasis on applied knowledge and especially related to biotechnology.
Bioinformatics - also classified as a branch of information technology (IT) it is the study, collection, and storage of genomic data
Biomathematics or Mathematical Biology - the study of biological processes through mathematics, with an emphasis on modeling.
Biomechanics - often considered a branch of medicine, the study of the mechanics of living beings, with an emphasis on applied use through artificial limbs, etc.
Biophysics - the study of biological processes through physics, by applying the theories and methods traditionally used in the physical sciences
Biotechnology - a new and sometimes controversial branch of biology that studies the manipulation of living matter, including genetic modification
Botany - the study of plants
Cell Biology - the study of the cell as a complete unit, and the molecular and chemical interactions that occur within a living cell.
Conservation Biology - the study of the preservation, protection, or restoration of the natural environment, natural ecosystems, vegetation, and wildlife
Cryobiology - the study of the effects of lower than normally preferred temperatures on living beings.
Developmental Biology - the study of the processes through which an organism develops, from zygote to full structure.
Ecology - the study of the ecosystem as a complete unit, with an emphasis on how species and groups of species interact with other living beings and non-living elements.
Entomology - the study of insects
Environmental Biology - the study of the natural world, as a whole or in a particular area, especially as affected by human activity
Epidemiology - a major component of public health research, it is the study of factors affecting the health and illness of populations
Ethology - the study of animal behavior.
Evolution or Evolutionary Biology - the study of the origin and decent of species over time
Genetics - the study of genes and heredity.
Herpetology - the study of reptiles (and amphibians?)
Histology - The study of cells and tissue, a microscopic branch of anatomy.
Ichthyology - the study of fish
Macrobiology - the study of biology on the level of the macroscopic individual (plant, animal, or other living being) as a complete unit.
Mammology - the study of mammals
Marine Biology - the study of ocean ecosystems, plants, animals, and other living beings.
Medicine - the study of the human body in health and disease, with allopathic medicine focusing on alleviating or curing the body from states of disease
Microbiology - the study of microscopic organisms (microorganisms) and their interactions with other living things
Molecular Biology - the study of biology and biological functions at the molecular level, some cross over with biochemistry
Mycology - the study of fungi
Neurobiology - the study of the nervous system, including anatomy, physiology, even pathology
Oceanography - the study of the ocean, including ocean life, environment, geography, weather, and other aspects influencing the ocean. See Marine Biology
Ornithology - the study of birds
Paleontology - the study of fossils and sometimes geographic evidence of prehistoric life
Pathobiology or pathology - the study of diseases, and the causes, processes, nature, and development of disease
Parisitology - the study of parasites and parasitism
Pharmacology - the study and practical application of preparation, use, and effects of drugs and synthetic medicines.
Physiology - the study of the functioning of living organisms and the organs and parts of living organisms
Phytopathology - the study of plant diseases
Pre-medicine - a college major that covers the general aspects of biology as well as specific classes relevant to the study of medicine
Virology - the study of viruses and some other virus-like agents, usually considered part of microbiology or pathology
Zoology - the study of animals and animal life, including classification, physiology, development, and behavior (See also Entomology, Ethology, Herpetology, Ichthyology, Mammology, Ornithology
Subscribe to:
Comments (Atom)
